Defective
Hip replacement
Smith & Nephew, depuy, Wright Medical, ZIMMER biomet & Stryker hip implant complications
Although hip replacements have been hailed as a groundbreaking remedy for joint problems, this advance has not been without complications. There have been a variety of harmful side effects that have been associated with this defective hip implants. Lawyers across the country are filing hip replacement lawsuits to get justice and compensation for victims.
Major manufacturers of hip replacement devices:
- DePuy Synthes
- Zimmer Biomet
- Stryker
- Wright Medical Technology and MicroPort Orthopedics, Inc.
- Smith & Nephew
Hip replacement lawsuits assert that victims endured pain and suffering, infection, dislocation, loosening, metallosis and or revision surgery after defective hip implant surgeries. Many victims had severe complications and injuries from a faulty hip implant.
There are several hip replacement models which are widely known to be defective and dangerous. These faulty hip replacement devices have been the cause of thousands of hip replacement lawsuits. Well over 29,000 hip implant lawsuits had been pursued in consolidated lawsuits. Hip replacement device manufacturers have ponied up more than $7 billion to reach settlements with victims.
Victims who filed Hip implant lawsuits often needed a 2nd surgery
Smith & Nephew’s Birmingham Hip Resurfacing (BHR) System
- Recall June 2015
- It was pulled from the hip replacement device market as a result of studies that indicated that the hip implant was caused a very high rate of problems and complications leading to many revision surgeries.
- Widely used between 2006 and 2015
- Manufacturer is Smith & Nephew
- Unbelievably high complication rate leading to revision surgeries.
- Complications: Pain, Instability of joint, Metallosis (corrosion), revision surgery, blood poisoning/ toxicity – Cobalt and Chronium, problems standing and walking, fracture bones, dislocation, Necrosis, decreased mobility, inflammation and more.
Smith and Nephew’s R3 Hip Replacement systems.
- “Smith & Nephew R3 Acetabular hip systems have been recalled due to their high failure rate coupled with reports of patients experiencing severe side effects, including metal poisoning and bone damage.” Device watch
- Smith & Nephew is a UK corporation. It has offices in Memphis, TN.
- “After discovering consumers were experiencing difficulty with the R3 Acetabular System, Smith & Nephew issued a market recall for the metal liner component of the devices. Consumers and surgeons found revision surgery was needed in a higher than expected rate with these systems and on June 1, 2012 the recall was initiated. R3 Acetabular Systems are still in use and the company recommends physicians carefully monitor patients to ensure the in-use devices are not malfunctioning.” Drug Dangers
Biomet Hip replacement lawsuits:
-
Name of consolidated MDL: In Re: Biomet M2A Magnum Hip Implant Products Liability Litigation
-
MDL 2391- Presiding Judge: Robert L. Miller, Jr.
- Northern District of Indiana
- “The cases in this litigation primarily involve alleged defects in Biomet’s M2a Magnum system of hip implant products. Plaintiffs’ claims focus upon the metal-on-metal design of the M2a Magnum system and the alleged propensity of the M2a Magnum devices to generate high levels of metal ions, cause metallosis in the surrounding tissue, and/or fail early.” Indiana District
- Read the transfer order
- There are currently 35 cases remaining in this MDL. At the zenith of this litigation there were 2883 lawsuits pending.
- “More than 2,800 lawsuits have been filed over Biomet’s M2a Magnum hip implants. The company agreed to settle most of the cases in 2014.” Drug watch
- “While the litigation was underway, orthopedic manufacturer Zimmer bought Biomet in 2014 for $13.4 billion. The new company, Zimmer Biomet, became one of the leaders in the hip replacement market in 2016.” Drug watch
-
November 25, 2020: “A federal judge in Missouri has signed off on a $21 million judgment against Zimmer Biomet (NYSE:ZBH) involving the company’s much-litigated M2a Magnum metal-on-metal hip implant. U.S. District Judge Stephen R. Clark’s sign-off yesterday of the award — $20 million to Mary Bayes and $1 million to her husband Philip Bayes — came a day after a federal jury in Iowa awarded $3.55 million to Lori Nicholson and her husband Willis for injuries sustained as a result of the M2A Magnum.”
Wright Medical Technology and MicroPort Orthopedics, Inc. consolidated Hip replacement lawsuit
-
Wright Profemur Hip Replacement: This is not the normal hip implant. The usual run of the mill hip implant replacement has a single femoral component. The Wright Profemur hip stem contains two modular pieces. These modular pieces make it possible for the implant to be adjustable for leg length. Nonetheless, complications with the modular design have lead to a bad reputation for the Wright Profemur system. There are many reports that the metal pieces rubbing against the other causes corrosion. This occurs during daily routine activity. In hip replacement lawsuits filed against Wright, victims assert that the femoral neck stem often fractures causing hip replacement failure.
-
MDL No. 2949, Eastern District of Arkansas Assigned to the Honorable Kristine G. Baker
-
“defects in the Wright Medical and Microport Profemur line of modular hip implants, which were offered in titanium and cobalt chromium alloys.” Id.
-
UNITED STATES JUDICIAL PANEL on MULTIDISTRICT LITIGATION – IN RE: PROFEMUR HIP IMPLANT PRODUCTS LIABILITY LITIGATION MDL No. 2949 TRANSFER ORDER
- “Plaintiffs contend that the modular devices are prone to micromovements that lead to fluid ingress into the bore, which leads to fretting and corrosion in the stem-neck junction, which in turn leads to metallosis and increased blood metal levels and, at times, fracture of the devices.” Id.
-
“Background: The cases concern alleged defects in the Wright Medical and Microport Profemur line of modular hip implants, which were offered in titanium and cobolt chromium alloys. On August 7, 2020, the United States Judicial Panel for Multidistrict Litigation (JPML) found that actions in this litigation involve common questions of fact. The JPML determined that centralization in the Eastern District of Arkansas will serve the convenience of the parties and witnesses and promote the just and efficient conduct of the litigation. The JPML transferred the matter to the Eastern District of Arkansas and, with the consent of the Court, assigned the cases to the Honorable Kristine G. Baker for coordinated or consolidated pretrial proceedings.” District Court
- Read the BRIEF IN SUPPORT OF PLAINTIFFS’ MOTION FOR TRANSFER OF ACTIONS PURSUANT TO 28 U.S.C. §1407
- MDL Court contact info: Tracy Washington, Courtroom Deputy, Tracy_Washington@ared.uscourts.gov 501-604-5424, James McCormack, Clerk of Court, 501-604-5351, Brady Hibbs, MDL Clerk- 501-604-5356 – mdl2949@ared.uscourts.gov
- 11/20/2020 order
- Stryker LFIT V40 Femoral Head Products Liability Litigation
- Indira Talwani (U.S. District Judge) MDL -2768 IN RE:
- https://www.consumersafety.org/medical-device-lawsuits/hip-replacement/
- History: In 2014, Stryker paid over $1 billion in settlement of thousands of Stryker hip replacement lawsuits. This Styyker faulty hip implant settlement was for Rejuvenate and ABG II hip replacement medical devices.
-
“In August of 2016, Stryker Corp recalled more than 42,000 of its LFIT V40 metal femoral head hip implant components. The company notified surgeons and health care facilities that the implants had a higher than expected rate of revision surgery due to a problem known as “taper lock” failure.Patients also experienced complications of the device including: Severe pain Loss of mobility Joint dislocation Metallosis or poisoning, Infection.” Drug Dangers
-
Stryker has been reaching settlements with victims for years.
-
Recalled product: “LFIT Anatomic V40 Femoral Head, Low Friction Ion Treatment, Sterile, 36 mm, REF 6260-9-236; Modular components designed to be locked onto a femoral hip stem trunnion during surgery for total hip replacement.” FDA
- Recalling Manufacturer: Stryker Howmedica Osteonics Corp.
325 Corporate Dr
Mahwah NJ 07430-2006 - Manufacturer reason for recall: “Stryker received several complaints describing incidence of harm secondary to taper lock failure for specific lots of numerous catalog numbers of LFIT Anatomic CoCr V40 Femoral Heads.” FDA
DePuy Pinnacle Hip Systems Lawsuits
- name of MDL: IN RE: DePUY ORTHOPAEDICS, INC., PINNACLE HIP IMPLANT PRODUCTS LIABILITY LITIGATION
- MDL No. 2244
- Northern District of Texas
- Honorable James E. Kinkeade presiding Justice
- A jury in Dallas awarded 6 victims who faulted Johnson & Johnson and its subsidiary DePuy Orthopaedics for severe complications from a Pinnacle hip replacement implant. Depuy is a subsidiary of Johnson and Johnson. The victims needed revision surgeries as a result of the defective medical device which was a metal on metal hip replacement. The huge verdict included 30 million in compensatory damages and 1 billion in punitive damages.
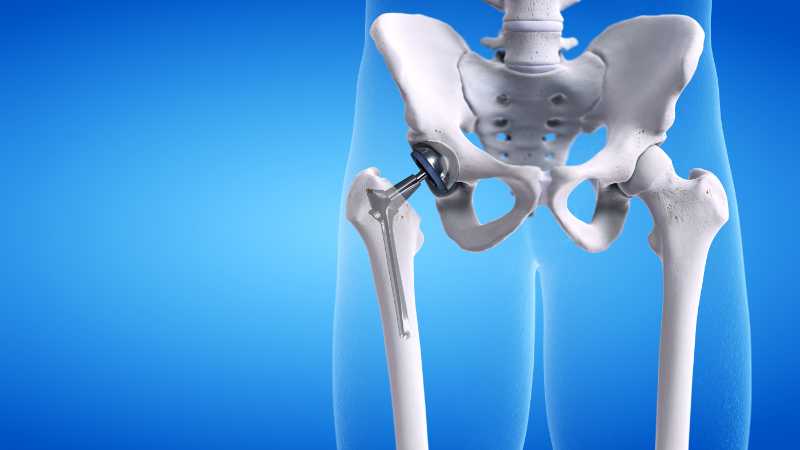
Symptoms of a defective hip replacement
Hip replacement complications are a serious matter. All of the problems that have been encountered with metal on metal hip replacement medical devices relate to the metal design of the prosthetic hip. The deleterious complications and side effects of the hip replacement medical devices have caused patients to incur further medical bills and additional surgeries. These problems have plagued hip replacement devices that have been manufactured by the two primary manufacturers of hip replacement products. There are legal remedies available for those who continue to suffer from the severe side effects of hip replacements. There are a number of open lawsuits against the manufacturers of these products. Many people are searching for information about: chromium metal, Metallosis, metal poisoning and cobalt poisoning. Other people are researching heavy metal toxicity symptoms. In any event, if you are desirous of filing a hip replacement lawsuit, contact a top hip replacement lawsuit lawyer.
Hip replacement complications
“Risks associated with hip replacement surgery may include:
- “Blood clots. Clots can form in your leg veins after surgery….”
- “Infection. Infections can occur at the site of your incision and in the deeper tissue near your new hip…”
- “Fracture. During surgery, healthy portions of your hip joint may fracture. Sometimes the fractures are so small that they heal on their own, but larger fractures may need to be corrected with wires, pins, and possibly a metal plate or bone grafts.”
- “Dislocation. Certain positions can cause the ball of your new joint to become dislodged, particularly in the first few months after surgery. If the hip dislocates, your doctor may fit you with a brace to keep the hip in the correct position. If your hip keeps dislocating, surgery is often required to stabilize it.”
- “Change in leg length. Your surgeon takes steps to avoid the problem, but occasionally a new hip makes one leg longer or shorter than the other…”
- “Loosening. Although this complication is rare with newer implants, your new joint may not become solidly fixed to your bone or may loosen over time, causing pain in your hip. Surgery might be needed to fix the problem.” Mayo Clinic
The manufacturers
WHAT YOU NEED TO KNOW ABOUT
DePuy and Stryker

1
Two of the main manufacturers of hip replacements are Stryker and DePuy. The two companies are competitors in the space. They have filed patent infringement lawsuits against each other. DePuy is a subsidiary of medical and pharmaceutical giant Johnson & Johnson. Stryker is also a major medical devices company in its own right. If you want to file a hip replacement lawsuit, contact one of the best hip replacement lawsuit attorneys in the United States.
As a result of the many devastating effects that recipients have suffered, there are a large number of lawsuits that have been filed against the manufacturers of hip replacement medical devices. Hip replacement lawsuits allege numerous different areas where the companies should be held legally liable. The lawsuits began to be filed in 2010, in the wake of the first recall issued by DePuy. Within a year after the recall, there were already 700 lawsuits filed in federal courts against DePuy. The manufacturer prevailed in many of the early cases relating to the metal-on-metal implant. However, the tide has recently shifted in favor of the plaintiffs in this particular litigation. Recently, plaintiffs have been racking up a string of victories at trial.
One of the more momentous cases in this class of litigation was filed in federal court in Texas. Six plaintiffs filed suit against DePuy for the complications and injuries that they sustained from their hip replacements. The plaintiffs sued under the theory that the metal-on-metal design of the artificial joint was defective. The defective design is what caused their injuries, and the lawsuit proceeded under a product liability theory. Specifically, the metal, which was designed to be stronger, instead caused the plaintiffs to get metallosis. The plaintiffs prevailed at trial when the case went to the jury. In what was a resounding victory, the six plaintiffs were awarded a total of $247 million by the jury..
2
3
Stryker has also had to defend numerous lawsuits that have been brought against it for the defective design of its product. There have been thousands of lawsuits that have been filed. Stryker attempted to defend the lawsuits, but unsuccessfully tried to persuade a federal court in Rhode Island to dismiss the case. After the court denied the motion to dismiss, Stryker entered settlement negotiations with the plaintiffs. In 2014, Stryker settled the class action for approximately $1.4 billion. This established a fund that has given plaintiffs as much as $600,000 in compensation for the injuries that they suffered.
If you have received a metal-on-metal hip replacement that was made by either Stryker or DePuy, you may be entitled to compensation for any side effects that you have experienced. If you have not already done so, you should immediately contact a hip replacement lawsuit attorney and learn your legal rights. You may be entitled to compensation. Each case has different facts so there is no guarantee that you will receive compensation.
The Problems
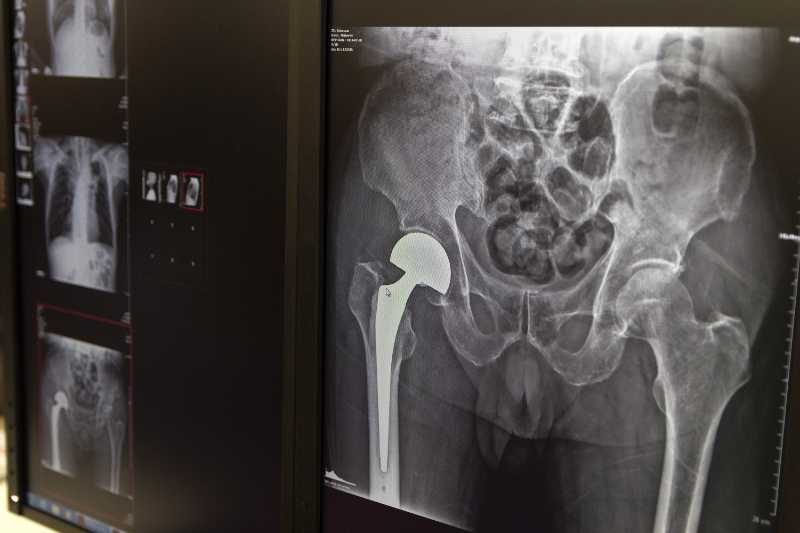
Metal-on-metal hips
A hip replacement involves the removal of the natural damaged joint and replacing it with a prosthetic device. There are several different types of hip replacements, including partial and total hip replacements. The actual prosthetic hip is made out of either metal or a combination of metal and plastic. The earliest prosthetic hips were made out of strictly metal. When a replacement hip is said to be metal-on-metal, it means that the ball and the socket are made out of titanium or steel.
highest rates of failure are found in the hip replacements that are metal-on-metal
The growing rate of hip replacements, combined with the insertion into the body of a device that contains metal has caused a variety of complications for many of the patients who have received these implants. The highest rates of failure are found in the hip replacements that are metal-on-metal. The failures have been so pronounced that these types of implants are no longer used for full hip replacements, but are not only used in hip resurfacing.
What is Metallosis?
“Metallosis is a rare, potentially fatal complication after arthroplasty, but is generally associated with metal-on-metal prosthetic devices, but it has also been described in non-metallic prostheses [3]. It is defined as aseptic fibrosis, local necrosis, or loosening of a device secondary to metal corrosion and release of wear debris [4], [5]. It occurs due to metallic erosion and release of metallic debris products, which induce massive local cytokines liberation from inflammatory cells [3], [6]. The systemic absorption of metallic particles can cause several clinical presentations depending on the predominant affected system. We present a case of metallosis, with a hemolytic anemia presentation in a patient with a non-metallic prosthesis, which occurred due to facture of the non-metallic component, leading to metal-metal contact. Exuberant radiological findings are described and their importance in surgical treatment planning is highlighted.” NCBI
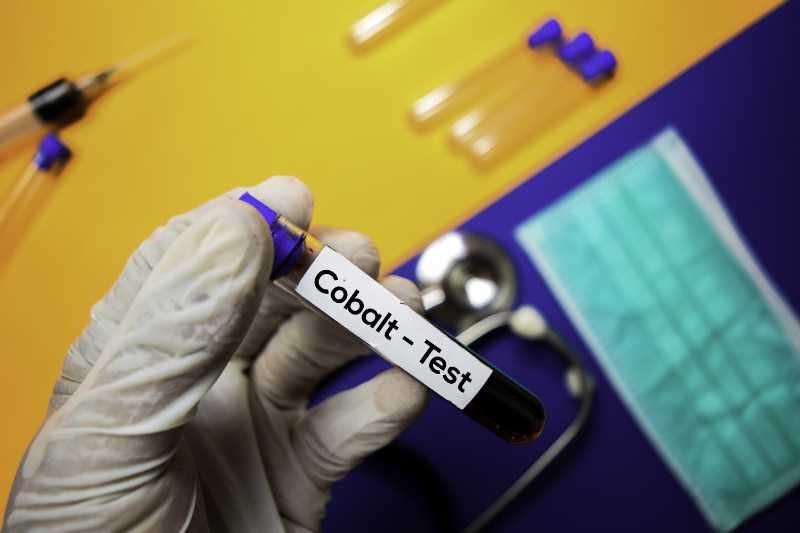
Metal Toxicity
When a patient has received a metal-on-metal hip replacement, it is the introduction of metal into the body which causes many adverse side effects. Metal-on-metal hip replacements have failed at a much higher rate than other types of prosthetic hips. The metal particles have had two primary effects on recipients. The first is that the metal particles get dissolved into the bloodstream and travel to and impact other parts of the body. The second major effect is that the metal inhibits the body’s ability to grow bone around the area that has received the implant. This is a necessary step for the body to heal and fully integrate the hip replacement. A hip replacement infection lawsuit may be an option for those suffering with side effects.
In practical terms, these two conditions mean that patients experience an unfortunate variety of side effects. At a minimum, many hip replacement patients have experienced pain that occurs many years after the procedure as the artificial joint fails. In addition, many patients have reported suffering infections from the metal. The most severe of the complications stem from the toxicity of the metal. Especially when a hip replacement contains cobalt, this can lead to poisoning in the blood. (metal poisoning and cobalt poisoning) This has caused chromosomal changes that could lead to heart failure, dementia, cancer and many other sever life-threatening issues. The side effects of this product have been so severe that, in 2010, DePuy was forced to issue a recall of its ASR XL Acetabular metal-on-metal hip replacement system. In addition, Stryker’s LFIT V40 Femoral Head was recalled in 2016, affecting products that were manufactured as early as 2006.
For more information about cobalt chrome and chromium ion visit:
Chromium and cobalt ion concentrations in blood and serum following various types of metal-on-metal hip arthroplasties
For more information about heavy metal toxicity visit here.
Get hip replacement picture here.
Sources: https://www.cdc.gov/nchs/products/databriefs/db186.htm
http://www.ajrr.net/images/annual_reports/AJRR-2017-Annual-Report—Final.pdf
